The Fifth Dose: Understanding the “Fifth Dose” in Mounjaro (Tirzepatide) KwikPen Use
Fifth dose – this information is to explain what the term means and in no way endorses the use, or promotes stepping outside manufacturer guidelines.
In the context of Mounjaro and the KwikPen, the term “Golden Dose” or “fifth dose” refers to the leftover medication that remains in the pen after administering the four pre-measured doses.
How Some Access the Fifth Dose ( aka Golden Dose)
Some may use a traditional syringe to extract 0.6ml of medication from the pen cartridge. While Monj doesn’t explicitly condemn this practice for its ability to retrieve an accurate amount. Neither the manufacturer nor healthcare professionals recommend it.
Alternatively, individuals may try to retrieve this leftover medication by breaking the KwikPen. After the fourth dose, this is done by twisting the pen until it locks, and forcing it until the lock breaks. This then allows the pen to turn and access the residual medication. However, this method poses additional safety risks as it can lead to inaccurate dosing. This happens because the process may damage the mechanism.
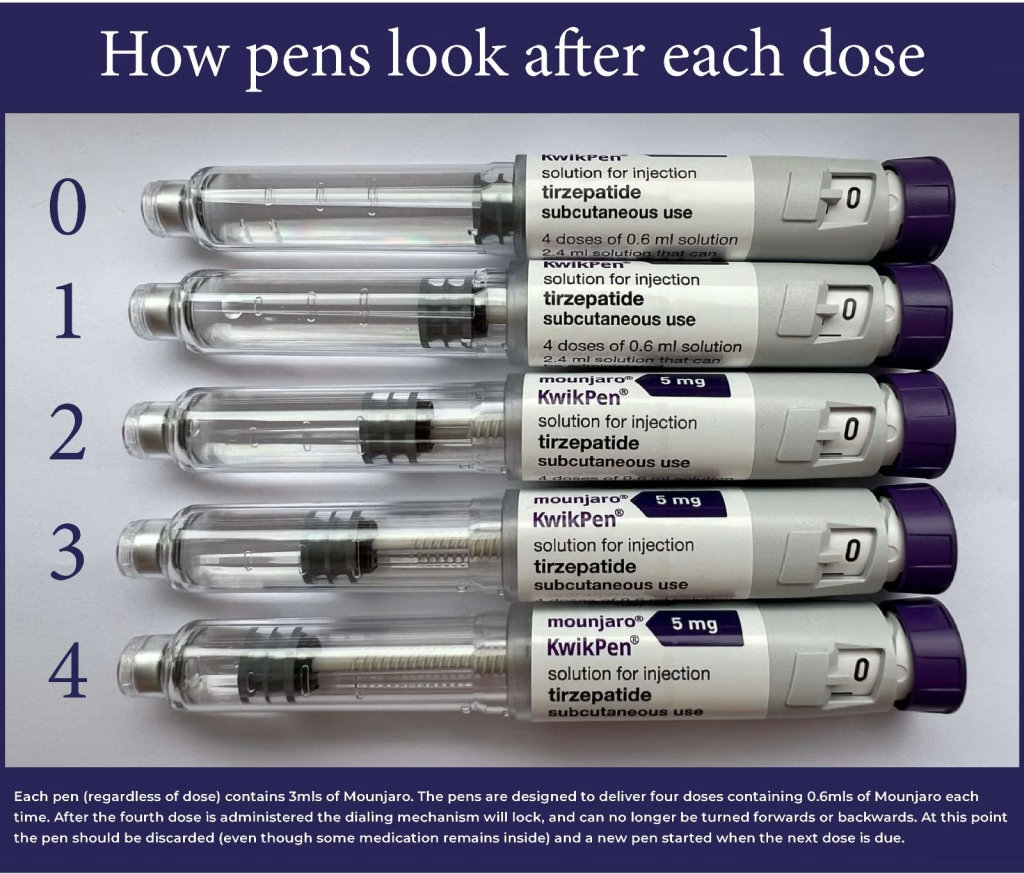
KwikPen and Doses
The Mounjaro KwikPen delivers four doses, with each dose containing a specific amount of medication. (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg, depending on the prescribed dosage). After completing the four doses, a small amount of residual medication, often referred to as “buffer liquid,” typically remains due to the design of the KwikPen.
The Fifth Dose / Golden Dose: Is It Really Leftover?
Although this residual liquid exists, you should not view it as a complete or accurate dose. Medical professionals caution against using it as a “fifth dose” due to the potential for incorrect dosing—either underdosing (insufficient medication) or overdosing (excessive medication). This could undermine the treatment’s effectiveness and increase the risk of side effects.
The leftover liquid is essentially “buffer liquid”. Intended to ensure the proper delivery of the full four doses due to the original design of the pen. Once the four doses have been used, guidelines recommend discarding the pen in a waste bin. Even if some liquid remains.
Please always dispose of the needles in a sharps container.
The Truth Behind the KwikPen Design (Thanks to a Top-tier Anonymous Pharmacist)
The KwikPen was originally developed as a pre-filled, disposable pen designed primarily for insulin administration. The Kwikpen has a total volume of 3ml (300 units). This design enabled patients to self-administer multiple doses using the same pen, providing convenience and flexibility in managing their medication. However, instead of creating a brand-new pen specifically for Mounjaro (tirzepatide), Eli Lilly chose to adapt the existing KwikPen design for this new medication. This adaptation made the production process more cost-effective, allowing the company to leverage established manufacturing systems and facilities. By reusing the original pen design, Eli Lilly avoided the lengthy and costly process of obtaining new safety approvals. Regulatory certifications required to create an entirely new pen would have also been costly. This strategic approach helped streamline production and brought Mounjaro to market more quickly, ensuring that patients had access to this popular weight loss and diabetes treatment without unnecessary delays.
Key Takeaway: The “Fifth Dose” is Leftover medication
Ultimately, the “fifth dose” is not a true, valid dose. It’s simply the leftover medication. It’s the leftover medication in the pen after the four prescribed doses have been administered. While we completely respect the choices of individuals, it’s important legally for Monj to note that medical professionals advise against using the leftover medication outside of its intended purpose.
As always, you should make any decision to deviate from general medication guidelines under the supervision of a qualified healthcare provider. Monj is not qualified to offer advice on this matter.