Thinking of Switching to Wegovy?
Are you thinking of switching to Wegovy from Mounjaro due to the price rises?
We’ve had reports and have seen some advertising encouraging patients to switch from Mounjaro to Wegovy, with price increases from September 2026, it seems like an easy decision.
We remain neutral and are only presenting clinical trial data for context to help you make a decision on data that’s available.
Changing medication based on cost alone is a decision that should be considered carefully. Reviewing the results from clinical trials helps give an idea of how these medicines performed on average in research settings.
This page summarises trial data for Wegovy (STEP-1 study) and Mounjaro (SURMOUNT-1 study), alongside recent regulatory updates. It is not medical advice, always consult a qualified healthcare professional before making any decisions.
Wegovy vs Mounjaro (72 weeks, STEP-1 & SURMOUNT-1)
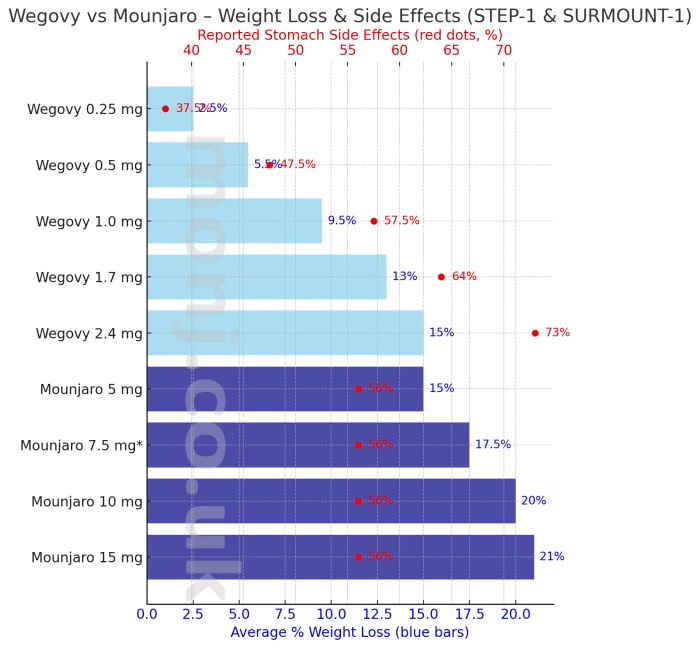
| Drug / Dose | Avg weight loss | 15%+ lost | 20%+ lost | Stomach issues |
|---|---|---|---|---|
| Wegovy 0.25 mg | ~2–3% | – | – | 35–40% |
| Wegovy 0.5 mg | ~5–6% | – | – | 45–50% |
| Wegovy 1.0 mg | ~9–10% | – | – | 57–58% |
| Wegovy 1.7 mg | ~13% | – | – | 64% |
| Wegovy 2.4 mg | ~15% | 50% | 32% | 73% |
| Mounjaro 5 mg | ~15% | 50% | 33% | 56% |
| Mounjaro 10 mg | ~20% | 67% | 50% | 56% |
| Mounjaro 15 mg | ~21% | 70% | 57% | 56% |
| Placebo | ~2–3% | 6% | 1% | 30% |
*7.5 mg is speculative: it was not directly studied in SURMOUNT-1, but the estimates here are based on trial patterns and clinical reports.
Important note:
This table is drawn specifically from the STEP-1 (semaglutide/Wegovy) and SURMOUNT-1 (tirzepatide/Mounjaro) clinical trials, each lasting 72 weeks. Other studies may report slightly different figures depending on patient groups, trial design, or follow-up periods.
We’ve chosen these trials because they are:
- The largest, most cited phase 3 studies for each drug in people without diabetes.
- Directly comparable (similar duration, placebo control, and large sample sizes).
- Widely referenced by clinicians, regulators, and researchers when discussing expected outcomes.
STEP-1 trial: Semaglutide in adults without diabetes (NEJM, 2021)
SURMOUNT-1 trial: Tirzepatide in adults without diabetes (NEJM, 2022)
What the data shows
- Wegovy 2.4 mg (full dose): Average 15% weight loss. Half of users lost at least 15% of their weight, and about 1 in 3 lost 20% or more. Stomach/gut side effects were reported by 73%.
- Mounjaro 10–15 mg: Average 20–21% weight loss. About 7 in 10 users lost 15% or more, and more than half lost 20% or more. Side effects occurred in 56% of users.
- Overall: In these clinical trials, Mounjaro showed greater weight loss and fewer gut side effects compared with Wegovy at the highest studied doses.
Considering cost?
For many people, cost is the main driver of switching. If you’re comparing full-dose Wegovy (2.4 mg) with lower or mid-dose Mounjaro, here’s a simplified view:
| Drug / Dose | Avg weight loss | 15%+ lost | 20%+ lost | Stomach issues |
|---|---|---|---|---|
| Wegovy 2.4 mg | ~15% | 50% | 32% | 73% |
| Mounjaro 5 mg | ~15% | 50% | 33% | 56% |
| Mounjaro 7.5 mg* | ~17–18% (est.) | ~60% (est.) | ~40–45% (est.) | ~56% (est.) |
*7.5 mg is speculative: it was not directly studied in SURMOUNT-1, but the estimates here are based on trial patterns and clinical reports.
Cost-related considerations:
- Wegovy 2.4 mg and Mounjaro 5 mg deliver similar average weight loss, but Wegovy had more reported side effects.
- Mounjaro 7.5 mg may well provide slightly more weight loss than Wegovy, with side effects similar to other Mounjaro doses (~56%).
- If switching mainly for price reasons, consider the trade-off between weight loss outcomes and side effects.
GPhC concerns about switching
The UK General Pharmaceutical Council (GPhC) has specifically raised concerns about how weight-management drugs are being supplied and promoted, especially when switching is encouraged for non-clinical reasons such as cost or advertising.
In plain terms, the regulator has warned that:
- Wegovy and Mounjaro are classed as “high-risk medicines.”
- Online pharmacies must now independently verify patient details (like height, weight, and BMI) before prescribing.
- The goal is to stop unsafe or inappropriate switching, such as patients being moved from one drug to another because of business or marketing pressures rather than medical need.
- Safe prescribing means decisions should be clinical, not commercial.
FAQ: Wegovy vs Mounjaro
Is Wegovy cheaper than Mounjaro?
Some pharmacies may push Wegovy as Mounjaro prices have risen, but switching based only on cost can affect results and side effects.
Do side effects go away over time?
In trials, nausea and gut issues were most common during dose escalation. Many people saw improvement over time, but not all did—hence the need for medical oversight.
Which works better: Wegovy or Mounjaro?
In STEP-1 and SURMOUNT-1, Mounjaro (10–15 mg) achieved 4+% greater average weight loss and fewer gut side effects than Wegovy (2.4 mg). But individual results can differ.
Why did the GPhC issue warnings?
The GPhC is concerned that some patients are being switched between medicines for commercial reasons rather than medical ones. Their updated rules mean pharmacies must ensure any switch is clinically appropriate and safe.
Important reminder
If you are considering starting, switching between Wegovy, Mounjaro, or any other weight-management treatment, always consult a qualified healthcare professional.